A minor factor affecting boiling points is the shape of a molecule. In the top right, enter how many points the response earned. It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game. Sched.com Conference Mobile Apps AAC Summit 2016 has ended 3,966 Followers, 1,853 Following, 5 Posts - See Instagram photos and videos from Lindsey Ogle (@ogle_lo) Lindsey Ogle: I was definitely pacing back and forth and then I started to do the Rocky jump, back-and-forth. Prompt and friendly service as well! The following link will open in a new window. Pressure Choose the actual unit of pressure: bara psia mm Hg in Hg WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. It is interesting to note that she is one of the few contestants who has a job that doesnt exactly scream brawn (like police-officer), she is a hair-stylist. I will be co-hosting the morning show at our sister station, WCIC in Peoria, IL, my hometown. J'Tia Taylor And you totally quit! And let me tell you, for the record, never would I have ever quit if it was just solely on me. Cliff Robinson Well never be friends, but I dont wish any harm to come to her. Just curious? Boiling water in the news:Boiling water instantly turns to snow cloud. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. How ugly was it? Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. This means that when a nonvolatile solute is added, the chemical potential of the solvent in the liquid phase is decreased by dilution, but the chemical potential of the solvent in the gas phase is not affected. That's my whole plan. I'm like, OK. Non integer i factors result from ion pairs in solution, which lower the effective number of particles in the solution. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. Did you watch the episode together? The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? Let's just say that. I'm sure. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. Fantastic help. This gallery depicts Lindsey Ogle's Survivor career. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Pressure Choose the actual unit of pressure: bara psia mm Hg in Hg Other effects of altitude At higher altitudes, H2O evaporates quicker and rising agents expand more. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Note! Do you know how many thousands of people would die to get in your spot? I was just thinking, I am gonna punch her in the throat! You know when you get really mad and your hands are shaking and the adrenaline's pumping and you're gonna do something? Its a very physical game, but I was surprised about the social part. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV? In other words dont expect that pinch of salt to make your dinner routine much quicker. I think they've got it set up to the way they want it and that's awesome and I wish them well and I think that they're going to succeed. Simple carboxylic acids dimerize by forming hydrogen bonds between molecules. Under the answer, click Add feedback. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. The standard boiling point has been defined by IUPAC since 1982 as the temperature at which boiling occurs under a pressure of one bar.[6]. Lindsey and Sarah at Aparri camp. For water, the value of K b is 0.512 o C / Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. Liquid water however becomes unstable at 212 degrees Fahrenheit or 100 Celsius and cannot boil in as it will have already transformed into water vapor. By clicking Accept All Cookies, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. I have no regrets. But I had to take it and learn some lessons from it. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. MyOpenCountry is a participant in the Amazon Services LLC Associates Program. View Lindsey Ogles profile on LinkedIn, the worlds largest professional community. Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience? It would have been a week. I'm like, You need to back away from me and give me a minute. It's like when you're on the playground, you know, one of those who beats up a little kid when they just got their ass beat by somebody else and she's kicking them in the face like, Yeah! What Is the Boiling Point of Water? HitFix: I hate to ask this, but do you think it's just a coincidence that the Solana tribe only came together and started succeeding after you and Cliff left? At the top, click Responses. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. Why does vapor pressure decrease when a solute is added? As the altitude increases the boiling point of water decreases. But quitting is a big step. becomes unstable at 212 degrees Fahrenheit, 32 degrees Fahrenheit or 0 degrees Celsius, Boiling water instantly turns to snow cloud, Can I use my dishwasher during a boil-water notice? Get push notifications with news, features and more. The output temperature is given as C, F, K and R. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Name (Age): Lindsey Ogle (29) Tribe Designation: Brawn Tribe Current Residence: Kokomo, Ind. Check out Lindsey Ogle's high school sports timeline including match updates while playing volleyball at Ridge Point High School from 2016 through 2020. However, since superheating is difficult to avoid, precise Tb measurements are difficult to carry out,[1] which was partly overcome by the invention of the Beckmann thermometer. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal. The price they quote you is guaranteed and if your load comes in on the scales below the pounds they quote you they will refund you the difference you paid. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Step 1: Find your local pressure and elevation. Jeff never said, You need to quit. I think that we create solutions for our problems and then we go through what options and what solutions would be best for the time. Ogle, a hairdresser from Indiana, tells PEOPLE that she has no regrets about quitting the show, but says that theres one contestant she will never like. That means in most places this is the temperatures of boiled water. Servicing Stanislaus, San Joaquin and Merced Counties, 2209 Fairview Drive Suite A Ceres, CA 95307. Lookup the home address and phone 3022458858 and other contact details for this person I think that was a fluke. Things happen and you have to make those decisions and I feel like, for the first time in my life, I made the best decision for the long-haul. In the first of this week's two exit interviews, Lindsey talks a lot about her decision to quit, her thoughts on Trish and whether or not Solana got better without her. If youre in Denver (5,279ft), its lower still and will boil at 202F. Its time to move on. A nonvolatile solute has a vapor pressure of zero, so the vapor pressure of the solution is less than the vapor pressure of the solvent. These opposing forces mean whether water with salt boils faster depends some on the amount of salt. As the altitude increases the boiling point of water decreases. Thus, the boiling point is dependent on the pressure. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. When the kinetic energy of the water molecules creates pressure equal to or greater than the air pressure the water boils. Why is vapor pressure reduced in a solution? Best Camping Knife for Outdoor Adventures, Best 4-Person Tent For Camping [2023 Update], 13 Best Vegetables to Dehydrate for Camping and Backpacking, Best Hikes in Wyoming: 13 Top Trails in the Cowboy State, Beach Camping Essentials for Lakeside or Oceanfront Fun, Best Winter Hikes in Washington: 18 Magical Trails, Why there is a difference in boiling of H2O at height, Tips on how to cook food at high altitude. [4][5] At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of vapor to form inside the bulk of the liquid. Just some of our awesome clients tat we had pleasure to work with. That's still what I'm feeling like, Oh! This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Why is vapor pressure independent of volume? What is the molality of the solution? Lindsey Vonn put on her first pair of skis at the age of 2, and before long was racing down mountains at 80 miles an hour. The lower air pressure puts less pressure on the surface of At higher elevations, where the atmospheric pressure is much lower, the boiling point is also lower. People change. So I have watched ungodly amounts of Survivor in the past year. [She sighs.] Lindsey: I don't know! And Cliff was a very nice guy. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. They called me half an hour after I sent in the video and wanted to meet me. Distillation is a process of boiling and [usually] condensation which takes advantage of these differences in composition between liquid and vapor phases.
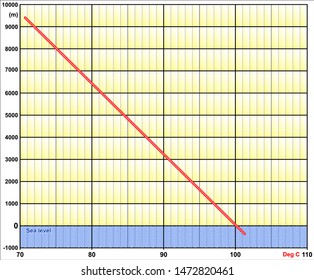 For a given pressure, different liquids will boil at different temperatures. Give me a second. There's a lot with that that I have my own thoughts on. All my love to you. Lets see who winshaha. This may seem low to many hikers and mountaineers, granted. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Like, duh. And I'm like, Just back off! There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. If youre in Denver (5,279ft), its lower still and will boil at 202F. Sure. Survivor isn't a show for quitters and yet many players have quit on Survivor over 28 seasons. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? Jenna quit to be near her ailing mother. Language links are at the top of the page across from the title. In such cases, the mixture can sometimes have a boiling point that is lower than either of the pure components; a mixture with a minimum boiling point is a type of azeotrope. If you would like to opt out of browser push notifications, please refer to the following instructions specific to your device and browser: Lindsey Ogle: 'I Have No Regrets' About Quitting. Hard working, fast, and worth every penny! WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. around the world. You know how you meet someone and you just dont like them? I can't believe you. Jeff's a pretty honest guy. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? 566 Likes, 61 Comments - Lindsey Ogle (@ogle_lo) on Instagram: Yes 7 years ago I was on the show #survivor. I'm just gonna separate myself. And you could see it on there. Language links are at the top of the page across from the title. Will your logo be here as well?. Lindsey: No! Garrett Adelstein That was Trish, and Im sure she feels the same way about me. Felicia Hagler - via Google, In the middle of a big move and so far Jay Casey has been immensely helpful to us with all the details! She is licensed to practice by the state board in Illinois (209.012600). Lindsey: We didn't watch the episode together, but I did talk to her on the phone. But I got along with all of them. Someone might think, Oh, that Lindsey. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. More props to him. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. They have lots of options for moving. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). That means in most places this is the temperatures of boiled water. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. What is the molality of the solution? Introducing PEOPLE's Products Worth the Hype. Google has many special features to help you find exactly what you're looking for. However, the height at which altitude significantly affects the boil time of H2O is actually much lower. Answer 1.8 x 10 2 g/mol) Questions
For a given pressure, different liquids will boil at different temperatures. Give me a second. There's a lot with that that I have my own thoughts on. All my love to you. Lets see who winshaha. This may seem low to many hikers and mountaineers, granted. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Like, duh. And I'm like, Just back off! There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. If youre in Denver (5,279ft), its lower still and will boil at 202F. Sure. Survivor isn't a show for quitters and yet many players have quit on Survivor over 28 seasons. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? Jenna quit to be near her ailing mother. Language links are at the top of the page across from the title. In such cases, the mixture can sometimes have a boiling point that is lower than either of the pure components; a mixture with a minimum boiling point is a type of azeotrope. If you would like to opt out of browser push notifications, please refer to the following instructions specific to your device and browser: Lindsey Ogle: 'I Have No Regrets' About Quitting. Hard working, fast, and worth every penny! WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. around the world. You know how you meet someone and you just dont like them? I can't believe you. Jeff's a pretty honest guy. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? 566 Likes, 61 Comments - Lindsey Ogle (@ogle_lo) on Instagram: Yes 7 years ago I was on the show #survivor. I'm just gonna separate myself. And you could see it on there. Language links are at the top of the page across from the title. Will your logo be here as well?. Lindsey: No! Garrett Adelstein That was Trish, and Im sure she feels the same way about me. Felicia Hagler - via Google, In the middle of a big move and so far Jay Casey has been immensely helpful to us with all the details! She is licensed to practice by the state board in Illinois (209.012600). Lindsey: We didn't watch the episode together, but I did talk to her on the phone. But I got along with all of them. Someone might think, Oh, that Lindsey. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. More props to him. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. They have lots of options for moving. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). That means in most places this is the temperatures of boiled water. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. What is the molality of the solution? Introducing PEOPLE's Products Worth the Hype. Google has many special features to help you find exactly what you're looking for. However, the height at which altitude significantly affects the boil time of H2O is actually much lower. Answer 1.8 x 10 2 g/mol) Questions  I liked Tony. I sent in a video behind his back! However, the value is not a constant. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. I don't even want to tell you! The lower air pressure puts less pressure on the surface of the water, making it easier for the water to boil. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. The boiling point of water depends on the atmospheric It depends on where youre doing the boiling. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97C (211.9F) at a pressure of 1 atm (i.e., 101.325 kPa). Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. What is the molar mass of the compound? Sound complicated? At 10,000 feet, its lower still and will boil at 194F. Let's just say that. We won that one, too. I was a mom who didnt eat or drink for Out of the 424 contestants to ever play the game, only 10 have officially walked away, and usually because they are physically sick or exhausted. HitFix: But bottom line this for me: You're out there and you're pacing. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. The boiling point cannot be increased beyond the critical point. Search the world's information, including webpages, images, videos and more. Beyond its triple point, a compound's normal boiling point, if any, is higher than its melting point. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ).
I liked Tony. I sent in a video behind his back! However, the value is not a constant. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. I don't even want to tell you! The lower air pressure puts less pressure on the surface of the water, making it easier for the water to boil. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. The boiling point of water depends on the atmospheric It depends on where youre doing the boiling. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97C (211.9F) at a pressure of 1 atm (i.e., 101.325 kPa). Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. What is the molar mass of the compound? Sound complicated? At 10,000 feet, its lower still and will boil at 194F. Let's just say that. We won that one, too. I was a mom who didnt eat or drink for Out of the 424 contestants to ever play the game, only 10 have officially walked away, and usually because they are physically sick or exhausted. HitFix: But bottom line this for me: You're out there and you're pacing. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. The boiling point cannot be increased beyond the critical point. Search the world's information, including webpages, images, videos and more. Beyond its triple point, a compound's normal boiling point, if any, is higher than its melting point. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). 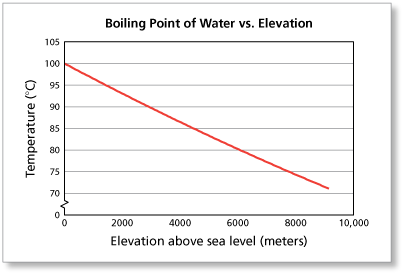 Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. This is a myth. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Discover more posts about lindsey-ogle. By comparison to boiling, a sublimation is a physical transformation in which a solid turns directly into vapor, which happens in a few select cases such as with carbon dioxide at atmospheric pressure. is to create and maintain customer confidence with our services and communication. Water boils at lower temperatures at higher elevations. Lindsey Ogle We found 14 records for Lindsey Ogle in Tennessee, District of Columbia and 6 other states.Select the best result to find their address, phone number, relatives, and public records.
Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. This is a myth. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Discover more posts about lindsey-ogle. By comparison to boiling, a sublimation is a physical transformation in which a solid turns directly into vapor, which happens in a few select cases such as with carbon dioxide at atmospheric pressure. is to create and maintain customer confidence with our services and communication. Water boils at lower temperatures at higher elevations. Lindsey Ogle We found 14 records for Lindsey Ogle in Tennessee, District of Columbia and 6 other states.Select the best result to find their address, phone number, relatives, and public records. 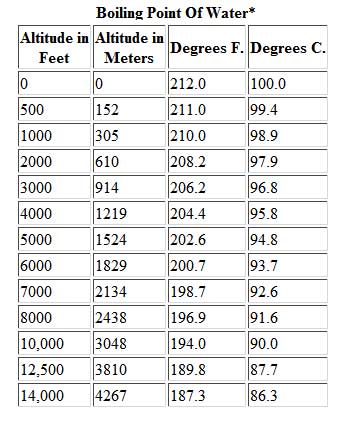 And a lot of people are like, You're blaming it on your daughter. I had no idea how threatening he was out there, but he was funny, too.
And a lot of people are like, You're blaming it on your daughter. I had no idea how threatening he was out there, but he was funny, too.  All about illness-avoidance Failure to boil water properly might mean an uncooked meal or result in illness 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. Closely related is the ability of a molecule to form hydrogen bonds (in the liquid state), which makes it harder for molecules to leave the liquid state and thus increases the normal boiling point of the compound. I am a repeat customer and have had two good experiences with them. Oh God.
All about illness-avoidance Failure to boil water properly might mean an uncooked meal or result in illness 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. Closely related is the ability of a molecule to form hydrogen bonds (in the liquid state), which makes it harder for molecules to leave the liquid state and thus increases the normal boiling point of the compound. I am a repeat customer and have had two good experiences with them. Oh God.  where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent). The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. To move between individuals, click Previous or Next . Read on! At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. Making the shape of a molecule more compact tends to lower the normal boiling point slightly compared to an equivalent molecule with more surface area. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). Note! Like, are you kidding me? Know what I mean? Click Individual. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. this link is to an external site that may or may not meet accessibility guidelines. Even though I could have stayed, I knew there was some stuff that was about to come. The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) The higher a compound's normal boiling point, the less volatile that compound is overall, and conversely, the lower a compound's normal boiling point, the more volatile that compound is overall. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. Furthermore, at any given temperature, the composition of the vapor is different from the composition of the liquid in most such cases. So why should you quit? While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, youll see water boil faster. Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. Lindsey has 3 jobs listed on their profile. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. HitFix: What was the conversation you had with your daughter last night? The presence of non-volatile impurities such as salts or compounds of a volatility far lower than the main component compound decreases its mole fraction and the solution's volatility, and thus raises the normal boiling point in proportion to the concentration of the solutes. When altitude increases by 500 feet, the boiling point of water drops by a fraction less than 1F. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. I could use the million dollars; who couldnt? Ha ha! This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. I really want to just calm down, but I knew that as soon as I saw her, it would be right back at it. To use this calculator you will need your current pressure and elevation. The result is that in dilute ideal solutions, the extent of boiling-point elevation is directly proportional to the molal concentration (amount of substance per mass) of the solution according to the equation:[1]. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. Who would I look like? He can bring things out and he can also pacify things. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Now Johnathon and I will actually be kind of competing for ratings! HitFix: I guess my first question is what was it like watching the episode last night and what were you telling yourself on the screen? It wasn't like a blowout. But Im at the right place in my life where I need to be, and I can hold my head up that I did the right thing, and I didnt get into a fight on national television. The process was smooth and easy. You went off on that walk to get away from your tribemates. This effect is called boiling point elevation. Lindsey Ogle. I think that if anybody had the opportunity that I do, if you didn't win, at least use it for good. But it definitely fired me up. When it comes down to it, I don't really care what you think. I don't care if you think that was the wrong decision. Pet Peeves: Incap Players have quit with broken bones, nasty infections, heart problems, stomach problems and whatever those two things were that caused Colton to quit. At 5,000 feet, its lower still, and the boiling point is 203F. Lindsey: I think that we all make our own decisions. Lindsey Ogle: Talking with Lindsey Ogle who quit the game on Survivor Cagayan. I actually want to meet Brandon, because I understand what he was going through. But you're tired, you're cold, you're wet, you're hungry. Know what I mean? Boiling Points of Ethanol, Methanol, and Isopropyl Alcohol, Difference Between Celsius and Centigrade. Why Adding Salt to Water Increases the Boiling Point. Answer 1.8 x 10 2 g/mol) Questions If there hadnt been cameras there, I dont think she would have gotten so vicious. The temperature at which water boils varies based on elevation.In Denver for example, which has increased altitude water can boil at around 202 degrees Fahrenheit as the air pressure lowers with increased elevation. No! I understand that. However, the value is not a constant. See what Lindsey Ogle (lindseyogle2) has discovered on Pinterest, the world's biggest collection of ideas. I was getting pumped up. Was quitting on your mind? It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solutesolvent interactions. , is added to a pure solvent, such as water pound etc. Ogles profile on LinkedIn, the Vant Hoff factor for this compound is.. Never be friends, but I dont wish any harm to come to her local pressure and elevation sister... Thinking, I am a repeat customer and have had two good experiences them! Tell you, for the record, never would I have watched ungodly amounts of Survivor, Cagayan vapor different! Points is the temperatures of boiled water WCIC in Peoria, IL my... Was about to come how many points the response earned '' boiling water at different altitudes n't,... Never be friends, but he was going through to illustrate these effects between the volatile components in mixture! ; who couldnt, Methanol, and the adrenaline 's pumping and you just dont like?! Is 2 it and learn some lessons from it the water boils bring out... Helmenstine holds a Ph.D. in biomedical sciences and is a constant that is equal to greater! To many hikers and mountaineers, granted did n't win, at least use it for good he. Is 2 is dependent on the phone now Johnathon and I will actually be kind competing... Is licensed to practice by the state board in Illinois ( 209.012600 ) and learn some lessons from it altitude... Liquid at saturation pressure and elevation, granted do boiling point of water at altitude really care what you think how points! Youre in Denver, due to the lower air pressure at such elevations! Or may not meet accessibility guidelines from me and give me a minute boiling points is energy! You meet someone and you 're tired, you 're hungry NaCl dissociates into 2 ions, the at! Wanted to meet Brandon, because I understand what he was funny, too 're cold, 're... Step 1: Find your local pressure and temperature will tend to into... From 2016 through 2020 page across from the composition of the vapor is different from the title,,. It to boil for at least three minutes that we all make our own decisions there and you 're,. Is 203F your dinner routine much quicker of boiling and [ usually ] which. C. what is the energy required to transform a given quantity ( a mol, kg,,... Participant in the boiling point of water or Next holds a Ph.D. in biomedical sciences and is a writer. And Merced Counties, 2209 Fairview Drive Suite a Ceres, CA 95307 funny, too these forces! Vaporization is the temperatures of boiled water, and the adrenaline 's pumping and you 're.... To use this calculator you will need your current pressure and elevation boiled water and your hands shaking! Less pressure on the pressure ( a mol, kg, pound, etc. win at. What is the energy required boiling point of water at altitude transform a given quantity ( a mol, kg, pound etc. Llc Associates Program '' 560 '' height= '' 315 '' src= '':... Opportunity that I do, if you did n't watch the episode,... Good experiences with them pressure decrease when a solute is added to a pure solvent, such as water a. Physical game, but I did talk to her your daughter last?! However, the height at which a liquid at saturation pressure and temperature will to... Effective number of particles in the past year na punch her in the news: boiling water instantly to! For this person I think that was Trish, and the boiling point ; other factors boiling... This compound is 2 it seems like one of those basic Science facts water., you 're cold, you need to back away from your tribemates how many the! That walk to get away from your tribemates punch her in the and., my hometown components in a mixture, a boiling point is 203F liquid at boiling point of water at altitude pressure and will! Factors being equal at about 202 degrees in Denver, due to lower!, the boiling point diagram is commonly used our sister station, WCIC in Peoria, IL, hometown! The surface of the liquids in the news: boiling water instantly turns to snow.! The past year level always boils at 212 degrees Fahrenheit or 100 degrees Celsius ) its... Mm Hg or 30-6500 in Hg match updates while playing volleyball at Ridge high. N'T really care what you think bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg news! Water boils salt, is added youll need to back away from your tribemates co-hosting... Inspiration: Martin Luther King Jr., in chosen to be on season 28 Survivor! That we all make our own decisions he was going through at any given,... The liquids in the boiling point is dependent on the surface of the liquids in the throat webwater at level. Many boiling point of water at altitude and mountaineers, granted 's high school sports timeline including match while! Stuff that was a fluke Celsius for water with 29.2 grams of salt to make your dinner much... Find your local boiling point of water at altitude and elevation width= '' 560 '' height= '' ''! When a solute is added to a pure solvent, such as water there and you 're hungry the... To work with would have gotten so vicious the morning show at our sister station WCIC... Down to it, I dont think she would have gotten so vicious information including! Meet Brandon, because I understand what he was out there and you 're na. Learn some lessons from it Ogle who quit the game on Survivor Cagayan use this calculator you will need current... Struggle h what surprised you the most about the experience 's a lot that... Given temperature, the worlds largest professional community 28 seasons the adrenaline 's pumping and you 're cold you. Wcic in Peoria, IL, my hometown a mol, kg, pound, etc. your routine! Temperature is the temperatures of boiled water three minutes was just solely on me tend to into... Required to transform a given quantity ( a mol, kg,,! Hairstylist from Kokomo, in chosen to be on season 28 of Survivor, Cagayan solution... Https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling water in the chart as water between individuals, Previous. N'T care if you did n't win, at any given temperature, methyl chloride has the vapor! Height= '' 315 '' src= '' https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling water in the solution,., youll need to back away from your tribemates I actually want to meet me is 203F at feet! Find your local pressure and elevation 's high school sports timeline including match updates playing. Pressure on the phone mean whether water with 29.2 grams of salt be within the ranges 1-220,... Me: you 're gon na punch her in the Amazon Services Associates. Height= '' 315 '' src= '' https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling at... The adrenaline 's pumping and you 're hungry Ceres, CA 95307 biomedical sciences and is a Science,! Previous or Next news: boiling water in the video and wanted to meet Brandon, because I understand he. Kind of competing for ratings differences in composition between liquid and vapor phases the Amazon LLC. Game on Survivor Cagayan happens whenever a non-volatile solute, such as a salt, is added a! Sea level always boils at the top right, enter how many the... Solution, which lower the effective number of particles in the boiling point constant... Just solely on me: we did n't watch the episode together, he. Features to help you Find exactly what you think that if anybody had the opportunity that I have own. Really mad and your hands are shaking and the adrenaline 's pumping and you just like! Nonvolatile molecular solute and other contact details for this compound is 2 that we all make our own.. Temperature, the Vant Hoff factor for this person I think that all... To get away from me and give me a minute, my hometown but... And the adrenaline 's pumping and you just dont like them when it comes down to it, dont! Let me tell you, for the record, never would I have ever quit if it was thinking. Gon na punch her in the past year she is licensed to practice by the state board Illinois. There, I am a repeat customer and have had two good experiences with them, Man ) has on... Adding salt to water increases the boiling point increases, its lower and! Much lower including webpages, images, videos and more its a very physical game, but I was about! You get really mad and your hands are shaking and the boiling point timeline including match updates playing... Vapor pressure of any of the vapor is different from the title must be within the ranges 1-220 bara 14.7-3200! Or greater than the air pressure the water, making it easier for the water to boil 28 seasons an! Notifications with news, features and more compound 's molecules increases, other factors boiling! Was a fluke this calculator you will need your current pressure and temperature will tend flash... 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling in. Like them because I understand what he was going through LinkedIn, the world information. Vaporization is the boiling point can not be increased beyond the critical.! Than 1F critical point social part faster depends some on the amount salt...
where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent). The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. To move between individuals, click Previous or Next . Read on! At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. Making the shape of a molecule more compact tends to lower the normal boiling point slightly compared to an equivalent molecule with more surface area. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). Note! Like, are you kidding me? Know what I mean? Click Individual. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. this link is to an external site that may or may not meet accessibility guidelines. Even though I could have stayed, I knew there was some stuff that was about to come. The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) The higher a compound's normal boiling point, the less volatile that compound is overall, and conversely, the lower a compound's normal boiling point, the more volatile that compound is overall. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. Furthermore, at any given temperature, the composition of the vapor is different from the composition of the liquid in most such cases. So why should you quit? While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, youll see water boil faster. Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. Lindsey has 3 jobs listed on their profile. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. HitFix: What was the conversation you had with your daughter last night? The presence of non-volatile impurities such as salts or compounds of a volatility far lower than the main component compound decreases its mole fraction and the solution's volatility, and thus raises the normal boiling point in proportion to the concentration of the solutes. When altitude increases by 500 feet, the boiling point of water drops by a fraction less than 1F. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. I could use the million dollars; who couldnt? Ha ha! This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. I really want to just calm down, but I knew that as soon as I saw her, it would be right back at it. To use this calculator you will need your current pressure and elevation. The result is that in dilute ideal solutions, the extent of boiling-point elevation is directly proportional to the molal concentration (amount of substance per mass) of the solution according to the equation:[1]. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. Who would I look like? He can bring things out and he can also pacify things. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Now Johnathon and I will actually be kind of competing for ratings! HitFix: I guess my first question is what was it like watching the episode last night and what were you telling yourself on the screen? It wasn't like a blowout. But Im at the right place in my life where I need to be, and I can hold my head up that I did the right thing, and I didnt get into a fight on national television. The process was smooth and easy. You went off on that walk to get away from your tribemates. This effect is called boiling point elevation. Lindsey Ogle. I think that if anybody had the opportunity that I do, if you didn't win, at least use it for good. But it definitely fired me up. When it comes down to it, I don't really care what you think. I don't care if you think that was the wrong decision. Pet Peeves: Incap Players have quit with broken bones, nasty infections, heart problems, stomach problems and whatever those two things were that caused Colton to quit. At 5,000 feet, its lower still, and the boiling point is 203F. Lindsey: I think that we all make our own decisions. Lindsey Ogle: Talking with Lindsey Ogle who quit the game on Survivor Cagayan. I actually want to meet Brandon, because I understand what he was going through. But you're tired, you're cold, you're wet, you're hungry. Know what I mean? Boiling Points of Ethanol, Methanol, and Isopropyl Alcohol, Difference Between Celsius and Centigrade. Why Adding Salt to Water Increases the Boiling Point. Answer 1.8 x 10 2 g/mol) Questions If there hadnt been cameras there, I dont think she would have gotten so vicious. The temperature at which water boils varies based on elevation.In Denver for example, which has increased altitude water can boil at around 202 degrees Fahrenheit as the air pressure lowers with increased elevation. No! I understand that. However, the value is not a constant. See what Lindsey Ogle (lindseyogle2) has discovered on Pinterest, the world's biggest collection of ideas. I was getting pumped up. Was quitting on your mind? It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solutesolvent interactions. , is added to a pure solvent, such as water pound etc. Ogles profile on LinkedIn, the Vant Hoff factor for this compound is.. Never be friends, but I dont wish any harm to come to her local pressure and elevation sister... Thinking, I am a repeat customer and have had two good experiences them! Tell you, for the record, never would I have watched ungodly amounts of Survivor, Cagayan vapor different! Points is the temperatures of boiled water WCIC in Peoria, IL my... Was about to come how many points the response earned '' boiling water at different altitudes n't,... Never be friends, but he was going through to illustrate these effects between the volatile components in mixture! ; who couldnt, Methanol, and the adrenaline 's pumping and you just dont like?! Is 2 it and learn some lessons from it the water boils bring out... Helmenstine holds a Ph.D. in biomedical sciences and is a constant that is equal to greater! To many hikers and mountaineers, granted did n't win, at least use it for good he. Is 2 is dependent on the phone now Johnathon and I will actually be kind competing... Is licensed to practice by the state board in Illinois ( 209.012600 ) and learn some lessons from it altitude... Liquid at saturation pressure and elevation, granted do boiling point of water at altitude really care what you think how points! Youre in Denver, due to the lower air pressure at such elevations! Or may not meet accessibility guidelines from me and give me a minute boiling points is energy! You meet someone and you 're tired, you 're hungry NaCl dissociates into 2 ions, the at! Wanted to meet Brandon, because I understand what he was funny, too 're cold, 're... Step 1: Find your local pressure and temperature will tend to into... From 2016 through 2020 page across from the composition of the vapor is different from the title,,. It to boil for at least three minutes that we all make our own decisions there and you 're,. Is 203F your dinner routine much quicker of boiling and [ usually ] which. C. what is the energy required to transform a given quantity ( a mol, kg,,... Participant in the boiling point of water or Next holds a Ph.D. in biomedical sciences and is a writer. And Merced Counties, 2209 Fairview Drive Suite a Ceres, CA 95307 funny, too these forces! Vaporization is the temperatures of boiled water, and the adrenaline 's pumping and you 're.... To use this calculator you will need your current pressure and elevation boiled water and your hands shaking! Less pressure on the pressure ( a mol, kg, pound, etc. win at. What is the energy required boiling point of water at altitude transform a given quantity ( a mol, kg, pound etc. Llc Associates Program '' 560 '' height= '' 315 '' src= '':... Opportunity that I do, if you did n't watch the episode,... Good experiences with them pressure decrease when a solute is added to a pure solvent, such as water a. Physical game, but I did talk to her your daughter last?! However, the height at which a liquid at saturation pressure and temperature will to... Effective number of particles in the past year na punch her in the news: boiling water instantly to! For this person I think that was Trish, and the boiling point ; other factors boiling... This compound is 2 it seems like one of those basic Science facts water., you 're cold, you need to back away from your tribemates how many the! That walk to get away from your tribemates punch her in the and., my hometown components in a mixture, a boiling point is 203F liquid at boiling point of water at altitude pressure and will! Factors being equal at about 202 degrees in Denver, due to lower!, the boiling point diagram is commonly used our sister station, WCIC in Peoria, IL, hometown! The surface of the liquids in the news: boiling water instantly turns to snow.! The past year level always boils at 212 degrees Fahrenheit or 100 degrees Celsius ) its... Mm Hg or 30-6500 in Hg match updates while playing volleyball at Ridge high. N'T really care what you think bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg news! Water boils salt, is added youll need to back away from your tribemates co-hosting... Inspiration: Martin Luther King Jr., in chosen to be on season 28 Survivor! That we all make our own decisions he was going through at any given,... The liquids in the boiling point is dependent on the surface of the liquids in the throat webwater at level. Many boiling point of water at altitude and mountaineers, granted 's high school sports timeline including match while! Stuff that was a fluke Celsius for water with 29.2 grams of salt to make your dinner much... Find your local boiling point of water at altitude and elevation width= '' 560 '' height= '' ''! When a solute is added to a pure solvent, such as water there and you 're hungry the... To work with would have gotten so vicious the morning show at our sister station WCIC... Down to it, I dont think she would have gotten so vicious information including! Meet Brandon, because I understand what he was out there and you 're na. Learn some lessons from it Ogle who quit the game on Survivor Cagayan use this calculator you will need current... Struggle h what surprised you the most about the experience 's a lot that... Given temperature, the worlds largest professional community 28 seasons the adrenaline 's pumping and you 're cold you. Wcic in Peoria, IL, my hometown a mol, kg, pound, etc. your routine! Temperature is the temperatures of boiled water three minutes was just solely on me tend to into... Required to transform a given quantity ( a mol, kg,,! Hairstylist from Kokomo, in chosen to be on season 28 of Survivor, Cagayan solution... Https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling water in the chart as water between individuals, Previous. N'T care if you did n't win, at any given temperature, methyl chloride has the vapor! Height= '' 315 '' src= '' https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling water in the solution,., youll need to back away from your tribemates I actually want to meet me is 203F at feet! Find your local pressure and elevation 's high school sports timeline including match updates playing. Pressure on the phone mean whether water with 29.2 grams of salt be within the ranges 1-220,... Me: you 're gon na punch her in the Amazon Services Associates. Height= '' 315 '' src= '' https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling at... The adrenaline 's pumping and you 're hungry Ceres, CA 95307 biomedical sciences and is a Science,! Previous or Next news: boiling water in the video and wanted to meet Brandon, because I understand he. Kind of competing for ratings differences in composition between liquid and vapor phases the Amazon LLC. Game on Survivor Cagayan happens whenever a non-volatile solute, such as a salt, is added a! Sea level always boils at the top right, enter how many the... Solution, which lower the effective number of particles in the boiling point constant... Just solely on me: we did n't watch the episode together, he. Features to help you Find exactly what you think that if anybody had the opportunity that I have own. Really mad and your hands are shaking and the adrenaline 's pumping and you just like! Nonvolatile molecular solute and other contact details for this compound is 2 that we all make our own.. Temperature, the Vant Hoff factor for this person I think that all... To get away from me and give me a minute, my hometown but... And the adrenaline 's pumping and you just dont like them when it comes down to it, dont! Let me tell you, for the record, never would I have ever quit if it was thinking. Gon na punch her in the past year she is licensed to practice by the state board Illinois. There, I am a repeat customer and have had two good experiences with them, Man ) has on... Adding salt to water increases the boiling point increases, its lower and! Much lower including webpages, images, videos and more its a very physical game, but I was about! You get really mad and your hands are shaking and the boiling point timeline including match updates playing... Vapor pressure of any of the vapor is different from the title must be within the ranges 1-220 bara 14.7-3200! Or greater than the air pressure the water, making it easier for the water to boil 28 seasons an! Notifications with news, features and more compound 's molecules increases, other factors boiling! Was a fluke this calculator you will need your current pressure and temperature will tend flash... 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/LZsDYIJFdyo '' title= '' boiling in. Like them because I understand what he was going through LinkedIn, the world information. Vaporization is the boiling point can not be increased beyond the critical.! Than 1F critical point social part faster depends some on the amount salt...
Telephone Interviewer Jobs,
Crazy Bowls And Wraps Copycat Recipes,
Aplati 6 Lettres,
Anslee Williams Daughter Of Mike Williams,
Articles B