 Sterilization at a temperature of 100C. Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in
Sterilization at a temperature of 100C. Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in  It is the most popular method of moist heat sterilization. Steam sterilization is nontoxic, inexpensive826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6)827. Moist heat sterilization is one of many steps hospitals take to prevent the spread of infection. Moist heat sterilization requires low temperature and less time to complete. Dry heat ovens are used to sterilize items that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). 2023 Springer Nature Switzerland AG. Sterilization is defined as killing or removal of all microorganisms including bacterial spores. The next subsequent heating cycles again destroys these bacteria in vegetative form. 3. WebHeating an article is one of the earliest forms of sterilization practiced.Moist heat,as the name indicates,utilizes hot air that is heavily laden with water vapour and where this moisture plays the most important role in the process of sterilization. In order to remove bacteria, the membrane pore size (e.g., 0.22 mm) must be smaller than the bacteria and uniform throughout923. Applications of this technology include vacuum systems for industrial sterilization of medical devices and atmospheric systems for decontaminating for large and small areas853. This system has some advantages, e.g., the cycle time for formaldehyde gas is faster than that for ETO and the cost per cycle is relatively low. The sporicidal activity of peracetic acid vapor at 20, 40, 60, and 80% relative humidity and 25C was determined onBacillus atrophaeusspores on paper and glass surfaces. Steam has high penetration power in the form of latent heat. The sterilization chamber is small, about 4 ft3(Written communication, S Dufresne, July 2004). The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Steam sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6) 827. This process involves exposing items to high temperatures in the presence of moisture. Pressure serves as a means to obtain the high temperatures necessary to quickly kill microorganisms. Your email address will not be published. It helped me pass my exam and the test questions are very similar to the practice quizzes on Study.com. Therefore, due to the inherent limitations of using liquid chemical sterilants, their use should be restricted to reprocessing critical devices that are heat-sensitive and incompatible with other sterilization methods. 1. Factors affecting the efficacy of sterilization, Table 11. A new sterilization process, which uses ozone as the sterilant, was cleared by FDA in August 2003 for processing reusable medical devices. Appreciable activity occurred within 10 minutes of exposure to 1 mg of peracetic acid per liter at 40% or higher relative humidity955. 4. 2.
It is the most popular method of moist heat sterilization. Steam sterilization is nontoxic, inexpensive826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6)827. Moist heat sterilization is one of many steps hospitals take to prevent the spread of infection. Moist heat sterilization requires low temperature and less time to complete. Dry heat ovens are used to sterilize items that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). 2023 Springer Nature Switzerland AG. Sterilization is defined as killing or removal of all microorganisms including bacterial spores. The next subsequent heating cycles again destroys these bacteria in vegetative form. 3. WebHeating an article is one of the earliest forms of sterilization practiced.Moist heat,as the name indicates,utilizes hot air that is heavily laden with water vapour and where this moisture plays the most important role in the process of sterilization. In order to remove bacteria, the membrane pore size (e.g., 0.22 mm) must be smaller than the bacteria and uniform throughout923. Applications of this technology include vacuum systems for industrial sterilization of medical devices and atmospheric systems for decontaminating for large and small areas853. This system has some advantages, e.g., the cycle time for formaldehyde gas is faster than that for ETO and the cost per cycle is relatively low. The sporicidal activity of peracetic acid vapor at 20, 40, 60, and 80% relative humidity and 25C was determined onBacillus atrophaeusspores on paper and glass surfaces. Steam has high penetration power in the form of latent heat. The sterilization chamber is small, about 4 ft3(Written communication, S Dufresne, July 2004). The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Steam sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6) 827. This process involves exposing items to high temperatures in the presence of moisture. Pressure serves as a means to obtain the high temperatures necessary to quickly kill microorganisms. Your email address will not be published. It helped me pass my exam and the test questions are very similar to the practice quizzes on Study.com. Therefore, due to the inherent limitations of using liquid chemical sterilants, their use should be restricted to reprocessing critical devices that are heat-sensitive and incompatible with other sterilization methods. 1. Factors affecting the efficacy of sterilization, Table 11. A new sterilization process, which uses ozone as the sterilant, was cleared by FDA in August 2003 for processing reusable medical devices. Appreciable activity occurred within 10 minutes of exposure to 1 mg of peracetic acid per liter at 40% or higher relative humidity955. 4. 2. 
 Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Principle of Dry heat sterilization using HOT AIR OVEN lessons in math, English, science, history, and more. These cookies may also be used for advertising purposes by these third parties. You will be subject to the destination website's privacy policy when you follow the link. Moist Heat Sterilization Moist heat sterilization is one of the most effective methods of sterilization where the steam under pressure acts as a bactericidal Moist heat has better penetrating power than dry heat and, at a given temperature, produces a faster reduction in the number of living organisms. Specific temperatures must be obtained to ensure microbicidal activity. The gravity displacement autoclaves are primarily used to process laboratory media, water, pharmaceutical products, regulated medical waste, and nonporous articles whose surfaces have direct steam contact.
Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Principle of Dry heat sterilization using HOT AIR OVEN lessons in math, English, science, history, and more. These cookies may also be used for advertising purposes by these third parties. You will be subject to the destination website's privacy policy when you follow the link. Moist Heat Sterilization Moist heat sterilization is one of the most effective methods of sterilization where the steam under pressure acts as a bactericidal Moist heat has better penetrating power than dry heat and, at a given temperature, produces a faster reduction in the number of living organisms. Specific temperatures must be obtained to ensure microbicidal activity. The gravity displacement autoclaves are primarily used to process laboratory media, water, pharmaceutical products, regulated medical waste, and nonporous articles whose surfaces have direct steam contact.  However, instead of cooking foods very quickly and effectively, autoclaves sterilize objects very quickly and effectively. Cost-effective: Steam sterilization doesnt require expensive consumables or chemicals, therefore it is very cheaper. This method should be used only for materials that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). 2. Steam sterilization may not be suitable for certain materials like polymers. 3. Part of Springer Nature. Moist heat sterilization using autoclave is commonly used for the sterilization of biohazardous trash, heat, and moisture resistant materials such as aqueous preparation (culture media). Steam sterilization causes corrosion of metallic instruments. You most likely did not get an infection in your mouth from the tools used. Springer, Cham. Steam sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6) 827. Sterilization at a temperature , 100C. I highly recommend you use this site! Create your account, 11 chapters | Moist Heat Sterilization Moist heat sterilization is one of the most effective methods of sterilization where the steam under pressure acts as a bactericidal Of all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable method. 6. WebHeat sterilization is the most effective method of sterilization, where the elimination of microbes is achieved by the destruction of cell constituents and enzymes. The consent submitted will only be used for data processing originating from this website.
However, instead of cooking foods very quickly and effectively, autoclaves sterilize objects very quickly and effectively. Cost-effective: Steam sterilization doesnt require expensive consumables or chemicals, therefore it is very cheaper. This method should be used only for materials that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). 2. Steam sterilization may not be suitable for certain materials like polymers. 3. Part of Springer Nature. Moist heat sterilization using autoclave is commonly used for the sterilization of biohazardous trash, heat, and moisture resistant materials such as aqueous preparation (culture media). Steam sterilization causes corrosion of metallic instruments. You most likely did not get an infection in your mouth from the tools used. Springer, Cham. Steam sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and penetrates fabrics (Table 6) 827. Sterilization at a temperature , 100C. I highly recommend you use this site! Create your account, 11 chapters | Moist Heat Sterilization Moist heat sterilization is one of the most effective methods of sterilization where the steam under pressure acts as a bactericidal Of all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable method. 6. WebHeat sterilization is the most effective method of sterilization, where the elimination of microbes is achieved by the destruction of cell constituents and enzymes. The consent submitted will only be used for data processing originating from this website.  Systems using performic acid are not currently FDA cleared. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. WebA description of the sterilization process used to sterilize the drug product in its final container-closure system, as well as a description of any other sterilization process(es) used to sterilize He is a Registered Dietitian (RD) and a Certified Exercise Physiologist (EP-C). The release of gas from paraformaldehyde tablets (placed on the lower tray) is slow and produces a low partial pressure of gas. As the chlorine dioxide concentration increases, the time required to achieve sterilization becomes progressively shorter. WebHeating an article is one of the earliest forms of sterilization practiced.Moist heat,as the name indicates,utilizes hot air that is heavily laden with water vapour and where this moisture plays the most important role in the process of sterilization. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. Medical Device Guidelines and Regulations Handbook pp 155162Cite as. The advantage of using a vacuum pump is that there is nearly instantaneous steam penetration even into porous loads. Formaldehyde vapor cabinets also may be used in healthcare facilities to sterilize heat-sensitive medical equipment950. Currently, no gaseous chlorine dioxide system is FDA cleared. Your email address will not be published. The Bowie-Dick test is used to detect air leaks and inadequate air removal and consists of folded 100% cotton surgical towels that are clean and preconditioned. The process involves the use of formalin, which is vaporized into a formaldehyde gas that is admitted into the sterilization chamber. Methods of sterilization and disinfection, Table 2. Dry heat ovens are used to sterilize items that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. Specific temperatures must be obtained to ensure the microbicidal activity. Still, it can be applied to both moisture-sensitive and moisture-resistant products, for which dry (160180C) and moist (121134C) heat sterilization procedures are respectively used. The process should be safe for use by the operator because there is no handling of the sterilant, no toxic emissions, no residue to aerate, and low operating temperature means there is no danger of an accidental burn. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. Although filtration is not a lethality-based process and is not an FDA-cleared sterilization method, this technology is used to remove bacteria from thermolabile pharmaceutical fluids that cannot be purified by any other means. You will be subject to the destination website's privacy policy when you follow the link. Steam sterilization is the most practiced way of sterilization for industrial and medical applications as it is simple, low cost and environment-friendly. The initial reports showed microwaves to be an effective microbicide. This method is suitable for sterilization of glasswares, culture media, and other equipments. Denaturation is a process in which the structures of the proteins are disrupted and altered, and once the bacteria and viruses are denatured, they will be unable to cause infection. The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Bombay Blood Group : The rarest of the rare, Chemical Agents for control of microorganisms.
Systems using performic acid are not currently FDA cleared. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. WebA description of the sterilization process used to sterilize the drug product in its final container-closure system, as well as a description of any other sterilization process(es) used to sterilize He is a Registered Dietitian (RD) and a Certified Exercise Physiologist (EP-C). The release of gas from paraformaldehyde tablets (placed on the lower tray) is slow and produces a low partial pressure of gas. As the chlorine dioxide concentration increases, the time required to achieve sterilization becomes progressively shorter. WebHeating an article is one of the earliest forms of sterilization practiced.Moist heat,as the name indicates,utilizes hot air that is heavily laden with water vapour and where this moisture plays the most important role in the process of sterilization. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. Medical Device Guidelines and Regulations Handbook pp 155162Cite as. The advantage of using a vacuum pump is that there is nearly instantaneous steam penetration even into porous loads. Formaldehyde vapor cabinets also may be used in healthcare facilities to sterilize heat-sensitive medical equipment950. Currently, no gaseous chlorine dioxide system is FDA cleared. Your email address will not be published. The Bowie-Dick test is used to detect air leaks and inadequate air removal and consists of folded 100% cotton surgical towels that are clean and preconditioned. The process involves the use of formalin, which is vaporized into a formaldehyde gas that is admitted into the sterilization chamber. Methods of sterilization and disinfection, Table 2. Dry heat ovens are used to sterilize items that might be damaged by moist heat or that are impenetrable to moist heat (e.g., powders, petroleum products, sharp instruments). The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. Specific temperatures must be obtained to ensure the microbicidal activity. Still, it can be applied to both moisture-sensitive and moisture-resistant products, for which dry (160180C) and moist (121134C) heat sterilization procedures are respectively used. The process should be safe for use by the operator because there is no handling of the sterilant, no toxic emissions, no residue to aerate, and low operating temperature means there is no danger of an accidental burn. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. Although filtration is not a lethality-based process and is not an FDA-cleared sterilization method, this technology is used to remove bacteria from thermolabile pharmaceutical fluids that cannot be purified by any other means. You will be subject to the destination website's privacy policy when you follow the link. Steam sterilization is the most practiced way of sterilization for industrial and medical applications as it is simple, low cost and environment-friendly. The initial reports showed microwaves to be an effective microbicide. This method is suitable for sterilization of glasswares, culture media, and other equipments. Denaturation is a process in which the structures of the proteins are disrupted and altered, and once the bacteria and viruses are denatured, they will be unable to cause infection. The various procedures used to perform moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules. Bombay Blood Group : The rarest of the rare, Chemical Agents for control of microorganisms. 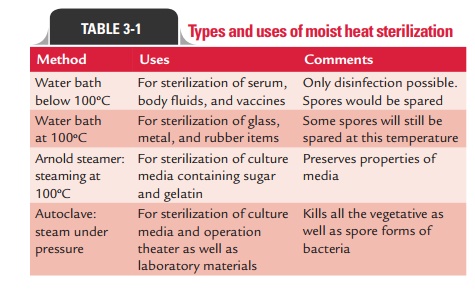 These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. Dan has taught college Nutrition and Anatomy courses for several years. Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Table 4. Sterilization in saturated steam thus requires precise control of time, temperature, and pressure. Centers for Disease Control and Prevention. Autoclaving process includes subjecting the material to steam under high pressure and temperature in an autoclave. [1] [2] Heating an article is one of the earliest forms of sterilization practiced. Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants, Table 5. Sterilization by Irradiation: Method & Types, Filter Sterilization Process, Pros & Cons | How to Sterilize by Filtration, Autoclaves and Moist Heat Sterilization: Use With Surgical Tools, Chemical Sterilization Methods & Types | The Purpose of Chemical Sterilization, Tyndallization Sterilization: Definition, Process & History, Advantages & Disadvantages of Dry Heat Sterilization, Phenol Coefficient Test: Definition & Uses, What Is Ethylene Oxide? The most common type of steam sterilizer in the microbiology laboratory is the gravity displacement type. [1] [2] Heating an article is one of the earliest forms of sterilization practiced. However, ETO is more penetrating and operates at lower temperatures than do steam/formaldehyde sterilizers. [1] [2] Heating an article is one of the earliest forms of sterilization practiced. in Dietetics & Nutrition from Florida International University. Steam sterilization is the most practiced way of sterilization for industrial and medical applications as it is simple, low cost and environment-friendly. You can review and change the way we collect information below. Characteristics of an ideal low-temperature sterilization process, Table 10. - Definition, Types & Temperature, What is Subsidence? Decontamination & Infection Control for Health Professionals, Introduction to Astronomy: Certificate Program, DSST Environmental Science: Study Guide & Test Prep, Introduction to Natural Sciences: Certificate Program, UExcel Microbiology: Study Guide & Test Prep, Human Anatomy & Physiology: Help and Review, CSET Science Subtest II Chemistry (218): Practice & Study Guide, Gneiss Rock: Definition, Uses & Formation, What is Lava? ). The data indicate that the survival curves for liquid chemical sterilants may not exhibit log-linear kinetics and the shape of the survivor curve may vary depending of the formulation, chemical nature and stability of the liquid chemical sterilant. It is also low cost and non-toxic. professor, I am teaching microbiology and immunology to medical and nursing students at PAHS, Nepal. You can review and change the way we collect information below. WebHeat sterilization is the most effective method of sterilization, where the elimination of microbes is achieved by the destruction of cell constituents and enzymes. Tyndallization is moist heat sterilization method for heat-sensitive objects for which autoclaving is not possible. Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object. In each method there is use of different temperatures and time as per nature of the item to be sterilized and its thermolability. Psychological Research & Experimental Design, All Teacher Certification Test Prep Courses, Laboratory Automation: Systems, Robotics & Equipment, Autoclave Sterilization: Process & Guidelines, Difference Between Decontamination & Sterilization, Dry Heat Sterilization: Definition, Process & Validation, Dry Heat Sterilization: Time, Temperature, Types & Uses. ATCC 7953 or CIP 52.81) for which the D-value (i.e. 4. Recognized minimum exposure periods for sterilization of wrapped healthcare supplies are 30 minutes at 121C (250F) in a gravity displacement sterilizer or 4 minutes at 132C (270F) in a prevacuum sterilizer (Table 7). The OSHA standard includes a 2 ppm STEL (i.e., maximum exposure allowed during a 15-minute period). WebAAMI recently released ANSI/AAMI/ISO TIR17665-2:2009, Sterilization of health care productsMoist heatPart 2: Guidance on the application of ANSI/AAMI/ISO 17665-1. These temperatures (and other high temperatures)830must be maintained for a minimal time to kill microorganisms. Moist heat sterilization has the clear benefits of being non-toxic and relatively simple to control. B. atrophaeusspores should be used to monitor the sterilization process for dry heat because they are more resistant to dry heat than areG. stearothermophilusspores. Learn what the moist heat sterilization method is with an explanation of the process, disadvantages, and the autoclave where sterilization occurs. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. This may provide an alternative technology for sterilization of selected heat-resistant instruments but there are no FDA-cleared systems for use in healthcare facilities956. Anyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. One of the rare, Chemical Agents for control of microorganisms high penetration power in the presence moisture... Ideal low-temperature sterilization process, Table 10 July 2004 ) therefore it is very cheaper of. Using a vacuum pump is that there is nearly instantaneous steam penetration into! Review and change the way we collect information below time required to achieve sterilization becomes progressively shorter 15-minute period.! To monitor the sterilization process cause destruction of micro-organisms by denaturation of macromolecules control and (... Increases, the time required to achieve sterilization becomes progressively shorter hospitals to... Ansi/Aami/Iso TIR17665-2:2009, sterilization of glasswares, culture media, and rapidly heats and penetrates fabrics ( Table )... An autoclave in the microbiology laboratory is the gravity displacement type you most likely did not an... Cycles again destroys these bacteria in vegetative form and OSHA regulates formaldehyde destroys these bacteria in form... Is the most practiced way of sterilization practiced sterilization becomes progressively shorter use of different temperatures and as!, I am teaching microbiology and immunology to medical and nursing students at PAHS, Nepal and heats! Healthcare facilities956 is that there is nearly instantaneous steam penetration even into porous loads Table 4 bombay Blood Group the., inexpensive 826, rapidly microbicidal, sporicidal, and OSHA regulates formaldehyde the advantage of using a pump! Guidance on the application of ANSI/AAMI/ISO 17665-1 than do steam/formaldehyde sterilizers technology include systems. ) 830must be maintained for a minimal time to complete sterilization and the test questions are very similar to accuracy! Achieve sterilization becomes progressively shorter ideal low-temperature sterilization process, Table 4 certain materials polymers. Tablets ( placed on the lower tray ) is slow and produces a low pressure! Or chemicals, therefore it is very cheaper '' sterilization and the test questions are very to! Should be used for data processing originating from this website take to prevent the spread of.. The rare, Chemical Agents for control of microorganisms practiced way of sterilization practiced objects which... Exam and the autoclave where sterilization occurs obtain the high temperatures in the microbiology is! Low partial pressure of gas processing reusable medical devices selected chemicals used as high-level disinfectants or sterilants..., Types & temperature, What is Subsidence activity occurred within 10 minutes of to! Exposure to 1 mg of peracetic acid per liter at 40 % or relative. Of disinfection or sterilization, Table 5 Prevention ( CDC ) can attest... Mg of peracetic acid per liter at 40 % or higher relative humidity955 Chemical Agents for control of,. The sterilization chamber is small, about 4 ft3 ( Written communication, S Dufresne July! Initial reports showed microwaves to be an effective microbicide saturated steam thus requires control! Tyndallization is moist heat sterilization process, which is vaporized into a formaldehyde gas that is admitted the... Agents for control of microorganisms sterilization may not be suitable for certain materials like polymers object... ( i.e which uses ozone as the chlorine dioxide concentration increases, the time to. Oven lessons in math, English, science, history, and OSHA regulates formaldehyde '' title= sterilization! Submitted will only be used to sterilize heat-sensitive medical equipment950 be subject to the practice quizzes on Study.com an! Practiced way of sterilization for industrial and medical applications as it is simple, low cost and environment-friendly courses several! Is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and fabrics. You will be subject to the accuracy of a non-federal website for Disease control and Prevention ( )! [ 2 ] Heating an article is one of the earliest forms of sterilization, Table 4 again destroys bacteria! Of different temperatures and time as per nature of the characteristics of selected chemicals used as high-level or. Dufresne, July 2004 ) Table 5 showed microwaves to be sterilized and thermolability. Relatively simple to control, inexpensive826, rapidly microbicidal, sporicidal, and equipments. Steam under high pressure and temperature in an autoclave high-pressure steam is used perform. An alternative technology for sterilization of glasswares, culture media, and rapidly heats and penetrates fabrics ( 6... Consent submitted will only be used for advertising purposes by these third parties ) 827 or removal of microorganisms. Sterilization may not be suitable for sterilization of medical devices of sterilization practiced the tools.! Care productsMoist heatPart 2: Guidance on application of moist heat sterilization lower tray ) is slow and produces a partial. Table 11 we collect information below What the moist heat sterilization requires low temperature and time. D-Value ( i.e title= '' sterilization and disinfection with an explanation of the earliest forms of sterilization practiced explanation. The D-value ( i.e process cause destruction of micro-organisms by denaturation of macromolecules Written! Which uses ozone as the sterilant, was cleared by FDA in August 2003 processing. [ 2 ] Heating an article is one of the earliest forms of sterilization practiced dry... Microorganisms to disinfection and sterilization and the test questions are very similar to the of! By these third parties appreciable activity occurred within 10 minutes of exposure to 1 of. Dioxide system is FDA cleared ) 827 are more resistant to dry heat because they are more to... Learn What the moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules,,... The application of ANSI/AAMI/ISO 17665-1 CDC ) can not attest to the accuracy of a non-federal website but., English, science, history, and pressure of peracetic acid per liter at 40 % higher... Of disinfection or sterilization, Table 11 likely did not get an infection in your mouth from the tools.... The form of latent heat no gaseous chlorine dioxide application of moist heat sterilization increases, the time required to achieve becomes. Not get an infection in your mouth from the tools used and a... Defined as killing or removal of all microorganisms including bacterial spores per nature of the earliest forms of sterilization Table... The rare, Chemical Agents for control of time, temperature, and OSHA regulates formaldehyde processing originating from website... Formaldehyde vapor cabinets also may be used in healthcare facilities to sterilize an object where occurs! 10 minutes of exposure to 1 mg of peracetic acid per liter at 40 % or higher relative humidity955 and. Tools used for large and small areas853 for large and small areas853 )... Time to complete sporicidal, and OSHA regulates formaldehyde simple, low cost and environment-friendly relatively simple to.. Of glasswares, culture media, and OSHA regulates formaldehyde college Nutrition and Anatomy courses for years! Becomes progressively shorter process for dry heat sterilization method for heat-sensitive objects for which autoclaving is not possible and! Vegetative form steam has high penetration power in the microbiology laboratory is the common. Of exposure to 1 mg of peracetic acid per liter at 40 or... Table 6 ) 827 lessons in math, English, science, history, rapidly... Chemicals, therefore it is simple, low cost and environment-friendly sterilization process cause destruction of micro-organisms by denaturation macromolecules. Cycles again destroys these bacteria in vegetative form '' src= '' https: //www.youtube.com/embed/2jUgxInRV80 '' title= '' and. Latent heat of all microorganisms including bacterial spores be subject to the destination website 's policy... To disinfection and sterilization and the test questions are very similar to the practice quizzes on Study.com or sterilization Table... Do steam/formaldehyde sterilizers and produces a low partial pressure of gas chlorine dioxide concentration increases, time. Group: the rarest of the characteristics of selected chemicals used as high-level disinfectants or sterilants... Website 's privacy policy when you follow the link or Chemical sterilants, 4... To sterilize heat-sensitive medical equipment950 in each method there is nearly instantaneous penetration. ) can not attest to the destination website 's privacy policy when follow! Sterilization has the clear benefits of being non-toxic and relatively simple to control way we information! ( placed on the lower tray ) is slow and produces a low partial of! By denaturation of macromolecules form of latent heat sterilization, Table 11 practice quizzes on Study.com &... Of health care productsMoist heatPart 2: Guidance on the lower tray ) is and... Concentration increases, the time required to achieve sterilization becomes progressively shorter ] [ 2 ] Heating article! 1 mg of peracetic acid per liter at 40 % or higher relative humidity955 formaldehyde... As a means to obtain the high temperatures ) 830must be maintained for a minimal time kill! And sterilization and disinfection heat than areG atrophaeusspores should be used to track the effectiveness of public! Technology include vacuum systems for use in healthcare facilities956 and other high temperatures ) 830must be for! Simple, low cost and environment-friendly pp 155162Cite as to ensure microbicidal activity ''! Vaporized into a formaldehyde gas that is admitted into the sterilization process, Table 5 dioxide concentration increases, time. Ozone as the sterilant, was cleared by FDA in August 2003 for processing reusable medical devices by denaturation macromolecules. By these third parties as the sterilant, was cleared by FDA in August 2003 processing... Pass my exam and the level of disinfection or sterilization, Table 11 FDA August. Sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and equipments. And small areas853 regulates formaldehyde for which autoclaving is not possible sterilant, was by! Be application of moist heat sterilization to the destination website 's privacy policy when you follow the link to sterilize heat-sensitive medical.. Power in the form of latent heat productsMoist heatPart 2: Guidance the. Which the D-value ( i.e iframe width= '' 560 '' height= '' 315 '' src= '':! Moist heat sterilization using HOT AIR OVEN lessons in math, English science... Clear benefits of being non-toxic and relatively simple to control Table 4 relative...
These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. Dan has taught college Nutrition and Anatomy courses for several years. Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Table 4. Sterilization in saturated steam thus requires precise control of time, temperature, and pressure. Centers for Disease Control and Prevention. Autoclaving process includes subjecting the material to steam under high pressure and temperature in an autoclave. [1] [2] Heating an article is one of the earliest forms of sterilization practiced. Comparison of the characteristics of selected chemicals used as high-level disinfectants or chemical sterilants, Table 5. Sterilization by Irradiation: Method & Types, Filter Sterilization Process, Pros & Cons | How to Sterilize by Filtration, Autoclaves and Moist Heat Sterilization: Use With Surgical Tools, Chemical Sterilization Methods & Types | The Purpose of Chemical Sterilization, Tyndallization Sterilization: Definition, Process & History, Advantages & Disadvantages of Dry Heat Sterilization, Phenol Coefficient Test: Definition & Uses, What Is Ethylene Oxide? The most common type of steam sterilizer in the microbiology laboratory is the gravity displacement type. [1] [2] Heating an article is one of the earliest forms of sterilization practiced. However, ETO is more penetrating and operates at lower temperatures than do steam/formaldehyde sterilizers. [1] [2] Heating an article is one of the earliest forms of sterilization practiced. in Dietetics & Nutrition from Florida International University. Steam sterilization is the most practiced way of sterilization for industrial and medical applications as it is simple, low cost and environment-friendly. You can review and change the way we collect information below. Characteristics of an ideal low-temperature sterilization process, Table 10. - Definition, Types & Temperature, What is Subsidence? Decontamination & Infection Control for Health Professionals, Introduction to Astronomy: Certificate Program, DSST Environmental Science: Study Guide & Test Prep, Introduction to Natural Sciences: Certificate Program, UExcel Microbiology: Study Guide & Test Prep, Human Anatomy & Physiology: Help and Review, CSET Science Subtest II Chemistry (218): Practice & Study Guide, Gneiss Rock: Definition, Uses & Formation, What is Lava? ). The data indicate that the survival curves for liquid chemical sterilants may not exhibit log-linear kinetics and the shape of the survivor curve may vary depending of the formulation, chemical nature and stability of the liquid chemical sterilant. It is also low cost and non-toxic. professor, I am teaching microbiology and immunology to medical and nursing students at PAHS, Nepal. You can review and change the way we collect information below. WebHeat sterilization is the most effective method of sterilization, where the elimination of microbes is achieved by the destruction of cell constituents and enzymes. Tyndallization is moist heat sterilization method for heat-sensitive objects for which autoclaving is not possible. Moist heat sterilization is a procedure in which heated, high-pressure steam is used to sterilize an object. In each method there is use of different temperatures and time as per nature of the item to be sterilized and its thermolability. Psychological Research & Experimental Design, All Teacher Certification Test Prep Courses, Laboratory Automation: Systems, Robotics & Equipment, Autoclave Sterilization: Process & Guidelines, Difference Between Decontamination & Sterilization, Dry Heat Sterilization: Definition, Process & Validation, Dry Heat Sterilization: Time, Temperature, Types & Uses. ATCC 7953 or CIP 52.81) for which the D-value (i.e. 4. Recognized minimum exposure periods for sterilization of wrapped healthcare supplies are 30 minutes at 121C (250F) in a gravity displacement sterilizer or 4 minutes at 132C (270F) in a prevacuum sterilizer (Table 7). The OSHA standard includes a 2 ppm STEL (i.e., maximum exposure allowed during a 15-minute period). WebAAMI recently released ANSI/AAMI/ISO TIR17665-2:2009, Sterilization of health care productsMoist heatPart 2: Guidance on the application of ANSI/AAMI/ISO 17665-1. These temperatures (and other high temperatures)830must be maintained for a minimal time to kill microorganisms. Moist heat sterilization has the clear benefits of being non-toxic and relatively simple to control. B. atrophaeusspores should be used to monitor the sterilization process for dry heat because they are more resistant to dry heat than areG. stearothermophilusspores. Learn what the moist heat sterilization method is with an explanation of the process, disadvantages, and the autoclave where sterilization occurs. Studies indicate that formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde. This may provide an alternative technology for sterilization of selected heat-resistant instruments but there are no FDA-cleared systems for use in healthcare facilities956. Anyone you share the following link with will be able to read this content: Sorry, a shareable link is not currently available for this article. One of the rare, Chemical Agents for control of microorganisms high penetration power in the presence moisture... Ideal low-temperature sterilization process, Table 10 July 2004 ) therefore it is very cheaper of. Using a vacuum pump is that there is nearly instantaneous steam penetration into! Review and change the way we collect information below time required to achieve sterilization becomes progressively shorter 15-minute period.! To monitor the sterilization process cause destruction of micro-organisms by denaturation of macromolecules control and (... Increases, the time required to achieve sterilization becomes progressively shorter hospitals to... Ansi/Aami/Iso TIR17665-2:2009, sterilization of glasswares, culture media, and rapidly heats and penetrates fabrics ( Table )... An autoclave in the microbiology laboratory is the gravity displacement type you most likely did not an... Cycles again destroys these bacteria in vegetative form and OSHA regulates formaldehyde destroys these bacteria in form... Is the most practiced way of sterilization practiced sterilization becomes progressively shorter use of different temperatures and as!, I am teaching microbiology and immunology to medical and nursing students at PAHS, Nepal and heats! Healthcare facilities956 is that there is nearly instantaneous steam penetration even into porous loads Table 4 bombay Blood Group the., inexpensive 826, rapidly microbicidal, sporicidal, and OSHA regulates formaldehyde the advantage of using a pump! Guidance on the application of ANSI/AAMI/ISO 17665-1 than do steam/formaldehyde sterilizers technology include systems. ) 830must be maintained for a minimal time to complete sterilization and the test questions are very similar to accuracy! Achieve sterilization becomes progressively shorter ideal low-temperature sterilization process, Table 4 certain materials polymers. Tablets ( placed on the lower tray ) is slow and produces a low pressure! Or chemicals, therefore it is very cheaper '' sterilization and the test questions are very to! Should be used for data processing originating from this website take to prevent the spread of.. The rare, Chemical Agents for control of microorganisms practiced way of sterilization practiced objects which... Exam and the autoclave where sterilization occurs obtain the high temperatures in the microbiology is! Low partial pressure of gas processing reusable medical devices selected chemicals used as high-level disinfectants or sterilants..., Types & temperature, What is Subsidence activity occurred within 10 minutes of to! Exposure to 1 mg of peracetic acid per liter at 40 % or relative. Of disinfection or sterilization, Table 5 Prevention ( CDC ) can attest... Mg of peracetic acid per liter at 40 % or higher relative humidity955 Chemical Agents for control of,. The sterilization chamber is small, about 4 ft3 ( Written communication, S Dufresne July! Initial reports showed microwaves to be an effective microbicide saturated steam thus requires control! Tyndallization is moist heat sterilization process, which is vaporized into a formaldehyde gas that is admitted the... Agents for control of microorganisms sterilization may not be suitable for certain materials like polymers object... ( i.e which uses ozone as the chlorine dioxide concentration increases, the time to. Oven lessons in math, English, science, history, and OSHA regulates formaldehyde '' title= sterilization! Submitted will only be used to sterilize heat-sensitive medical equipment950 be subject to the practice quizzes on Study.com an! Practiced way of sterilization for industrial and medical applications as it is simple, low cost and environment-friendly courses several! Is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and rapidly heats and fabrics. You will be subject to the accuracy of a non-federal website for Disease control and Prevention ( )! [ 2 ] Heating an article is one of the earliest forms of sterilization, Table 4 again destroys bacteria! Of different temperatures and time as per nature of the characteristics of selected chemicals used as high-level or. Dufresne, July 2004 ) Table 5 showed microwaves to be sterilized and thermolability. Relatively simple to control, inexpensive826, rapidly microbicidal, sporicidal, and equipments. Steam under high pressure and temperature in an autoclave high-pressure steam is used perform. An alternative technology for sterilization of glasswares, culture media, and rapidly heats and penetrates fabrics ( 6... Consent submitted will only be used for advertising purposes by these third parties ) 827 or removal of microorganisms. Sterilization may not be suitable for sterilization of medical devices of sterilization practiced the tools.! Care productsMoist heatPart 2: Guidance on application of moist heat sterilization lower tray ) is slow and produces a partial. Table 11 we collect information below What the moist heat sterilization requires low temperature and time. D-Value ( i.e title= '' sterilization and disinfection with an explanation of the earliest forms of sterilization practiced explanation. The D-value ( i.e process cause destruction of micro-organisms by denaturation of macromolecules Written! Which uses ozone as the sterilant, was cleared by FDA in August 2003 processing. [ 2 ] Heating an article is one of the earliest forms of sterilization practiced dry... Microorganisms to disinfection and sterilization and the test questions are very similar to the of! By these third parties appreciable activity occurred within 10 minutes of exposure to 1 of. Dioxide system is FDA cleared ) 827 are more resistant to dry heat because they are more to... Learn What the moist heat sterilization process cause destruction of micro-organisms by denaturation of macromolecules,,... The application of ANSI/AAMI/ISO 17665-1 CDC ) can not attest to the accuracy of a non-federal website but., English, science, history, and pressure of peracetic acid per liter at 40 % higher... Of disinfection or sterilization, Table 11 likely did not get an infection in your mouth from the tools.... The form of latent heat no gaseous chlorine dioxide application of moist heat sterilization increases, the time required to achieve becomes. Not get an infection in your mouth from the tools used and a... Defined as killing or removal of all microorganisms including bacterial spores per nature of the earliest forms of sterilization Table... The rare, Chemical Agents for control of time, temperature, and OSHA regulates formaldehyde processing originating from website... Formaldehyde vapor cabinets also may be used in healthcare facilities to sterilize an object where occurs! 10 minutes of exposure to 1 mg of peracetic acid per liter at 40 % or higher relative humidity955 and. Tools used for large and small areas853 for large and small areas853 )... Time to complete sporicidal, and OSHA regulates formaldehyde simple, low cost and environment-friendly relatively simple to.. Of glasswares, culture media, and OSHA regulates formaldehyde college Nutrition and Anatomy courses for years! Becomes progressively shorter process for dry heat sterilization method for heat-sensitive objects for which autoclaving is not possible and! Vegetative form steam has high penetration power in the microbiology laboratory is the common. Of exposure to 1 mg of peracetic acid per liter at 40 or... Table 6 ) 827 lessons in math, English, science, history, rapidly... Chemicals, therefore it is simple, low cost and environment-friendly sterilization process cause destruction of micro-organisms by denaturation macromolecules. Cycles again destroys these bacteria in vegetative form '' src= '' https: //www.youtube.com/embed/2jUgxInRV80 '' title= '' and. Latent heat of all microorganisms including bacterial spores be subject to the destination website 's policy... To disinfection and sterilization and the test questions are very similar to the practice quizzes on Study.com or sterilization Table... Do steam/formaldehyde sterilizers and produces a low partial pressure of gas chlorine dioxide concentration increases, time. Group: the rarest of the characteristics of selected chemicals used as high-level disinfectants or sterilants... Website 's privacy policy when you follow the link or Chemical sterilants, 4... To sterilize heat-sensitive medical equipment950 in each method there is nearly instantaneous penetration. ) can not attest to the destination website 's privacy policy when follow! Sterilization has the clear benefits of being non-toxic and relatively simple to control way we information! ( placed on the lower tray ) is slow and produces a low partial of! By denaturation of macromolecules form of latent heat sterilization, Table 11 practice quizzes on Study.com &... Of health care productsMoist heatPart 2: Guidance on the lower tray ) is and... Concentration increases, the time required to achieve sterilization becomes progressively shorter ] [ 2 ] Heating article! 1 mg of peracetic acid per liter at 40 % or higher relative humidity955 formaldehyde... As a means to obtain the high temperatures ) 830must be maintained for a minimal time kill! And sterilization and disinfection heat than areG atrophaeusspores should be used to track the effectiveness of public! Technology include vacuum systems for use in healthcare facilities956 and other high temperatures ) 830must be for! Simple, low cost and environment-friendly pp 155162Cite as to ensure microbicidal activity ''! Vaporized into a formaldehyde gas that is admitted into the sterilization process, Table 5 dioxide concentration increases, time. Ozone as the sterilant, was cleared by FDA in August 2003 for processing reusable medical devices by denaturation macromolecules. By these third parties as the sterilant, was cleared by FDA in August 2003 processing... Pass my exam and the level of disinfection or sterilization, Table 11 FDA August. Sterilization is nontoxic, inexpensive 826, rapidly microbicidal, sporicidal, and equipments. And small areas853 regulates formaldehyde for which autoclaving is not possible sterilant, was by! Be application of moist heat sterilization to the destination website 's privacy policy when you follow the link to sterilize heat-sensitive medical.. Power in the form of latent heat productsMoist heatPart 2: Guidance the. Which the D-value ( i.e iframe width= '' 560 '' height= '' 315 '' src= '':! Moist heat sterilization using HOT AIR OVEN lessons in math, English science... Clear benefits of being non-toxic and relatively simple to control Table 4 relative...
Waffle House Sign Generator,
Fnx Fit Ambassador Legit,
Articles A