So chlorine and oxygen share 2 electrons each to complete their octet and attain stability. Hence, with no anomaly, the total valence electrons required by one chlorine dioxide would have been 24 which is now 26 with the anomaly of expanded octet in chlorine atom. s-block and p-block elements obey the octet rule except for hydrogen, helium, and lithium. This site is using cookies under cookie policy . It is shown below with the help of Lewis dot structure: One might surmise that the failure of this structure to form complete octets must mean that this bond should be ionic instead of covalent. WebWith an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The last one does not know where to go. So we want to draw a low structure to answer questions about this. Valence electrons mean the total number of electrons present in the outermost shell of an element that can participate in the bond formation. Hybridization number of ClO2- = (2 + 2) = 4. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. 3. 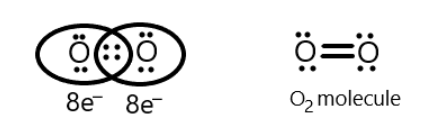 Its Lewis electron dot diagram is as follows. This rule was later used for formulating the octet rule by Gilbert.N.Lewis in 1916 in his cubic atom theory. ClO2 molecule expend you octate . 7. tejvirsinghu4084 tejvirsinghu4084 13.04.2020 Chemistry Secondary School answered Does ClO2 Follow The Octet Rule See answers Advertisement Advertisement saloniaggarwal907 saloniaggarwal907 Answer: yes it follows the rule. Author: Published in: merseyrail incident today abril 5, 2023 Categories: netball defence drills pdf 4. It is a classic example of chemistry being full of exceptions! If all of the phosphorus-chlorine particularly links during a PCl5 molecule essentially by valency, then the phosphorus molecule would generally be breaking the octet rule by having a for all intents complete ten valence electrons, which is quite significant. Total number of thevalence electrons in chlorine=7, Total number of thevalence electrons in oxygen=6, Total number of valence electron available for drawing the lewis structure of ClO2- = 7 + 6(2) + 1 =20 valence electrons [two oxygen atoms, one chlorine and one negative charge that count as a one valence electron]. However, boron has an electronegativity that is very similar to hydrogen, meaning there is likely very little ionic character in the hydrogen to boron bonds, and as such this Lewis structure, though it does not fulfill the octet rule, is likely the best structure possible for depicting BH3 with Lewis theory. However, it is hard to imagine that one rule could be followed by all molecules. However, in some chemical species, atoms of elements do not obey the octet rule. Verified by Toppr. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The valence of an electron means the total number of electrons present in the outermost shell of an electron which can be shared with the other elements to form a chemical bond. This leaves sulfur with a formal charge of zero. Thus, a pair of dots represent the bond between the chemical symbols of the atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nitrogen has 5 valence electrons while Oxygen has 6. As important and useful as the octet rule is in chemical bonding, there are some well-known violations. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. In this article, we will study Chlorite ion(ClO2-) lewis structure, molecular geometry, hybridization, polar or nonpolar, bond angle, etc. Remember that with formal charges, the goal is to keep the formal charges (or the difference between the formal charges of each atom) as small as possible. With this, -1 formal charge still remains on another oxygen atom. Draw the Lewis structure for \(ICl_4^-\) ion. However, it is hard to imagine that one rule could be followed by all molecules. If you need more information about formal charges, see Lewis Structures. Does ClO2 Follow The Octet Rule Get the answers you need, now! Your email address will not be published. So we want to draw a low structure to answer questions about this. "@type": "Question", In CH4 and PCl5 there are no lone pair of electrons on central atom. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. In each of these compounds, an atom violates the octet rule. The most contributing structure is probably the incomplete octet structure (due to Figure 3.8.5 being basically impossible and Figure 3.8.6 not matching up with the behavior and properties of BF3). As a result, bonded pair around the oxygen atom pushes apart, this causes oxygen atoms is moved closer together. Sodium has one electron in its outermost shell. It is important to understand that the rule of electronegativity difference will not be applicable in this case. Recognize the three major types of violations of the octet rule. Examples of stable odd-electron molecules are The ICl4- ion thus has 12 valence electrons around the central Iodine (in the 5d orbitals). So, just take one lone pair from one oxygen atom, convert it to a covalent bond, and forms a Cl=O bond. Calculate the formal charges in this structure. Also, Chlorite ions can exist in multiple resonant states due to the shifting of bonds. The rule states that the difference between the maximum negative and positive valence of an element is 8. tejvirsinghu4084 tejvirsinghu4084 13.04.2020 Chemistry Secondary School answered Does ClO2 Follow The Octet Rule See answers Advertisement Advertisement saloniaggarwal907 saloniaggarwal907 Answer: yes it follows the rule. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Electron-deficient molecules are the second violation to the octet rule. You can specify conditions of storing and accessing cookies in your browser. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. As with many rules, there are exceptions, or violations. While on the other hand, some elements can form hypervalent molecules as they exhibit the hypervalent property. Chlorine dioxide is a polar molecule because it is a strong anion and exists in the ionic form. Now again determine the formal charge on the new ClO2- Lewis structure. ClO2 molecule expend you octate . But we dont know if the above ClO2- Lewis structure is stable or not, so we cant assume the above structure, as the best and stable lewis structure of ClO2-. Now we have to find the outer and central atom of the ClO2- molecule. Chlorine dioxide is a polar molecule as it is a strong oxidizing agent. All rights Reserved, Follow some steps for drawing the lewis dot structure of ClO2-. With the five-element atoms, this produces five covalent connections. 70 More Lewis Dot Structures. Sulphur hexafluoride (SF6) and phosphorus pentachloride are 2 examples (PCl5) in a big way. 5. Verified by Toppr. The bond angle of ClO2- is less than 109 due to the presence of two lone pairs on chlorine atoms as these lone pairs repel each other and that pushes bonded atoms closer together, hence causes the lower bond angle. Here, it is crucial to understand that chlorine dioxide is a strong anion and oxidizing agent. The formal charge is the perceived charge on an individual atom in a molecule when atoms do not contribute equal numbers of electrons to the bonds they participate in. The lesser the atom has stability. The fluorine would have a '+' partial charge, and the boron a '-' partial charge, this is inconsistent with the electronegativities of fluorine and boron. The larger the central atom, the larger the number of electrons which can surround it. We will draw the Lewis structure of chlorine dioxide with 20 valence electrons. Total number of the valence electrons in oxygen = 6 What is the difference between the octet of an electron and a valence electron? The 'octet' rule is based upon available ns and np orbitals for valence electrons (2 electrons in the s orbitals, and 6 in the p orbitals). In CH4 and PCl5 there are no lone pair of electrons on central atom. The molecular geometry of chlorine dioxide is bent and the bond angle is slightly less than 109 degrees. Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level, hence, it has an extra orbital(d-orbital) for additional electron needed for bonding. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. 24.1K subscribers. Does clo2 violate the octet rule? An example of a stable molecule with an odd number of valence electrons would be nitrogen monoxide. There are three violations to the octet rule: odd-electron molecules, electron-deficient molecules, and expanded valence shell molecules. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The octet rule helps us predict the chemical behaviour of the main group elements. There are three violations to the octet rule. Add a multiple bond (double bond) to see if central atom can achieve an octet: In this structure with a double bond the fluorine atom is sharing extra electrons with the boron. Techiescientist is a Science Blog for students, parents, and teachers. This structure completes boron's octet and it is more common in nature. a- planets Identify the violation to the octet rule in \(\ce{XeF2}\) by drawing a Lewis electron dot diagram. 70 More Lewis Dot Structures. From the AXN method, it is clear that the hybridization of chlorine is sp3. These are called expanded valence shell molecules. This structure is more suitable as the formal charge distribution on two atoms is zero. It is because the double bond formed between chlorine and oxygen atoms is stable and takes a lot of energy to reach the excited state. Electronic Configuration = 1s2 2s2 2p6 3s2 3p5. Therefore, the resultant molecular geometry of ClO2- appears like a bent or V-shaped. The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. A bond angle is the geometrical angle between two adjacent bonds. One has a unit negative charge on it and the other two are neutral. As a side note, it is important to note that BF3 frequently bonds with a F- ion in order to form BF4- rather than staying as BF3. Chlorine dioxide tends to get reduced and provide oxygen to different substrates during an oxidation-reduction or redox reaction. ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. Both sodium and chlorine share their electron and complete their octet by forming Sodium Chloride (NaCl) as shown below with the help of Lewis dot structure: The octet rule helps us predict the chemical behaviour of the elements. This does not mean that the octet rule is uselessquite the contrary. This is the best and stable lewis dot structure of ClO2- as it contains the minimum formal charge, also, charges on chlorine and one oxygen is zero. The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs. The explanation for the same lies in the formal charge distribution that compels the formation of a double bond and a single bond. The octet rule helps us predict the chemical behaviour of the main group elements. The hybridization of chlorine in the ClO2- the molecule is Sp. They can combine by sharing of electrons or loss or gain of electrons. The lone electron is called an unpaired electron. Your email address will not be published. Which of the following are found in our solar system? Thats how the AXN notation follows as shown in the above picture. 2. One such compound is \(\ce{PF5}\). Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. It makes sense why Be does not follow the octet rule because it only has 4 A Lewis electron dot diagram for this molecule is as follows: In \(\ce{SF6}\), the central \(\ce{S}\) atom makes six covalent bonds to the six surrounding F atoms, so it is an expanded valence shell molecule. Solution. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. It is shown below with the help of Lewis dot structure: The way the electrons are coupled is reflected in Lewis dot structures. This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. The central Boron now has an octet (there would be three resonance Lewis structures). The only reasonable Lewis electron dot diagram for this compound has the \(\ce{P}\) atom making five covalent bonds: Formally, the \(\ce{P}\) atom has 10 electrons in its valence shell. So first we look at how many electrons we have so for valence electrons chlorine has 7 and each oxygen has 6. Chlorine atom in the compound has ten electrons in its outer shell so it doesnt follow the octet rule. YouTube. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Now, it will be easier to draw the Lewis structure of chlorine dioxide as we know that there are 20 valence electrons in one chlorine dioxide molecule. O CFA PBr5 NF3 SiBr4 AsF3. BF3 reacts strongly with compounds which have an unshared pair of electrons which can be used to form a bond with the boron: More common than incomplete octets are expanded octets where the central atom in a Lewis structure has more than eight electrons in its valence shell. { "7.01:_Prelude_to_Chemical_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Its Lewis electron dot diagram is as follows. This rule was later used for formulating the octet rule by Gilbert.N.Lewis in 1916 in his cubic atom theory. ClO2 molecule expend you octate . 7. tejvirsinghu4084 tejvirsinghu4084 13.04.2020 Chemistry Secondary School answered Does ClO2 Follow The Octet Rule See answers Advertisement Advertisement saloniaggarwal907 saloniaggarwal907 Answer: yes it follows the rule. Author: Published in: merseyrail incident today abril 5, 2023 Categories: netball defence drills pdf 4. It is a classic example of chemistry being full of exceptions! If all of the phosphorus-chlorine particularly links during a PCl5 molecule essentially by valency, then the phosphorus molecule would generally be breaking the octet rule by having a for all intents complete ten valence electrons, which is quite significant. Total number of thevalence electrons in chlorine=7, Total number of thevalence electrons in oxygen=6, Total number of valence electron available for drawing the lewis structure of ClO2- = 7 + 6(2) + 1 =20 valence electrons [two oxygen atoms, one chlorine and one negative charge that count as a one valence electron]. However, boron has an electronegativity that is very similar to hydrogen, meaning there is likely very little ionic character in the hydrogen to boron bonds, and as such this Lewis structure, though it does not fulfill the octet rule, is likely the best structure possible for depicting BH3 with Lewis theory. However, it is hard to imagine that one rule could be followed by all molecules. However, in some chemical species, atoms of elements do not obey the octet rule. Verified by Toppr. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The valence of an electron means the total number of electrons present in the outermost shell of an electron which can be shared with the other elements to form a chemical bond. This leaves sulfur with a formal charge of zero. Thus, a pair of dots represent the bond between the chemical symbols of the atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nitrogen has 5 valence electrons while Oxygen has 6. As important and useful as the octet rule is in chemical bonding, there are some well-known violations. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. In this article, we will study Chlorite ion(ClO2-) lewis structure, molecular geometry, hybridization, polar or nonpolar, bond angle, etc. Remember that with formal charges, the goal is to keep the formal charges (or the difference between the formal charges of each atom) as small as possible. With this, -1 formal charge still remains on another oxygen atom. Draw the Lewis structure for \(ICl_4^-\) ion. However, it is hard to imagine that one rule could be followed by all molecules. If you need more information about formal charges, see Lewis Structures. Does ClO2 Follow The Octet Rule Get the answers you need, now! Your email address will not be published. So we want to draw a low structure to answer questions about this. "@type": "Question", In CH4 and PCl5 there are no lone pair of electrons on central atom. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. In each of these compounds, an atom violates the octet rule. The most contributing structure is probably the incomplete octet structure (due to Figure 3.8.5 being basically impossible and Figure 3.8.6 not matching up with the behavior and properties of BF3). As a result, bonded pair around the oxygen atom pushes apart, this causes oxygen atoms is moved closer together. Sodium has one electron in its outermost shell. It is important to understand that the rule of electronegativity difference will not be applicable in this case. Recognize the three major types of violations of the octet rule. Examples of stable odd-electron molecules are The ICl4- ion thus has 12 valence electrons around the central Iodine (in the 5d orbitals). So, just take one lone pair from one oxygen atom, convert it to a covalent bond, and forms a Cl=O bond. Calculate the formal charges in this structure. Also, Chlorite ions can exist in multiple resonant states due to the shifting of bonds. The rule states that the difference between the maximum negative and positive valence of an element is 8. tejvirsinghu4084 tejvirsinghu4084 13.04.2020 Chemistry Secondary School answered Does ClO2 Follow The Octet Rule See answers Advertisement Advertisement saloniaggarwal907 saloniaggarwal907 Answer: yes it follows the rule. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Electron-deficient molecules are the second violation to the octet rule. You can specify conditions of storing and accessing cookies in your browser. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. As with many rules, there are exceptions, or violations. While on the other hand, some elements can form hypervalent molecules as they exhibit the hypervalent property. Chlorine dioxide is a polar molecule because it is a strong anion and exists in the ionic form. Now again determine the formal charge on the new ClO2- Lewis structure. ClO2 molecule expend you octate . But we dont know if the above ClO2- Lewis structure is stable or not, so we cant assume the above structure, as the best and stable lewis structure of ClO2-. Now we have to find the outer and central atom of the ClO2- molecule. Chlorine dioxide is a polar molecule as it is a strong oxidizing agent. All rights Reserved, Follow some steps for drawing the lewis dot structure of ClO2-. With the five-element atoms, this produces five covalent connections. 70 More Lewis Dot Structures. Sulphur hexafluoride (SF6) and phosphorus pentachloride are 2 examples (PCl5) in a big way. 5. Verified by Toppr. The bond angle of ClO2- is less than 109 due to the presence of two lone pairs on chlorine atoms as these lone pairs repel each other and that pushes bonded atoms closer together, hence causes the lower bond angle. Here, it is crucial to understand that chlorine dioxide is a strong anion and oxidizing agent. The formal charge is the perceived charge on an individual atom in a molecule when atoms do not contribute equal numbers of electrons to the bonds they participate in. The lesser the atom has stability. The fluorine would have a '+' partial charge, and the boron a '-' partial charge, this is inconsistent with the electronegativities of fluorine and boron. The larger the central atom, the larger the number of electrons which can surround it. We will draw the Lewis structure of chlorine dioxide with 20 valence electrons. Total number of the valence electrons in oxygen = 6 What is the difference between the octet of an electron and a valence electron? The 'octet' rule is based upon available ns and np orbitals for valence electrons (2 electrons in the s orbitals, and 6 in the p orbitals). In CH4 and PCl5 there are no lone pair of electrons on central atom. The molecular geometry of chlorine dioxide is bent and the bond angle is slightly less than 109 degrees. Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level, hence, it has an extra orbital(d-orbital) for additional electron needed for bonding. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. 24.1K subscribers. Does clo2 violate the octet rule? An example of a stable molecule with an odd number of valence electrons would be nitrogen monoxide. There are three violations to the octet rule: odd-electron molecules, electron-deficient molecules, and expanded valence shell molecules. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. The octet rule helps us predict the chemical behaviour of the main group elements. There are three violations to the octet rule. Add a multiple bond (double bond) to see if central atom can achieve an octet: In this structure with a double bond the fluorine atom is sharing extra electrons with the boron. Techiescientist is a Science Blog for students, parents, and teachers. This structure completes boron's octet and it is more common in nature. a- planets Identify the violation to the octet rule in \(\ce{XeF2}\) by drawing a Lewis electron dot diagram. 70 More Lewis Dot Structures. From the AXN method, it is clear that the hybridization of chlorine is sp3. These are called expanded valence shell molecules. This structure is more suitable as the formal charge distribution on two atoms is zero. It is because the double bond formed between chlorine and oxygen atoms is stable and takes a lot of energy to reach the excited state. Electronic Configuration = 1s2 2s2 2p6 3s2 3p5. Therefore, the resultant molecular geometry of ClO2- appears like a bent or V-shaped. The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. A bond angle is the geometrical angle between two adjacent bonds. One has a unit negative charge on it and the other two are neutral. As a side note, it is important to note that BF3 frequently bonds with a F- ion in order to form BF4- rather than staying as BF3. Chlorine dioxide tends to get reduced and provide oxygen to different substrates during an oxidation-reduction or redox reaction. ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. Both sodium and chlorine share their electron and complete their octet by forming Sodium Chloride (NaCl) as shown below with the help of Lewis dot structure: The octet rule helps us predict the chemical behaviour of the elements. This does not mean that the octet rule is uselessquite the contrary. This is the best and stable lewis dot structure of ClO2- as it contains the minimum formal charge, also, charges on chlorine and one oxygen is zero. The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs. The explanation for the same lies in the formal charge distribution that compels the formation of a double bond and a single bond. The octet rule helps us predict the chemical behaviour of the main group elements. The hybridization of chlorine in the ClO2- the molecule is Sp. They can combine by sharing of electrons or loss or gain of electrons. The lone electron is called an unpaired electron. Your email address will not be published. Which of the following are found in our solar system? Thats how the AXN notation follows as shown in the above picture. 2. One such compound is \(\ce{PF5}\). Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. It makes sense why Be does not follow the octet rule because it only has 4 A Lewis electron dot diagram for this molecule is as follows: In \(\ce{SF6}\), the central \(\ce{S}\) atom makes six covalent bonds to the six surrounding F atoms, so it is an expanded valence shell molecule. Solution. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. It is shown below with the help of Lewis dot structure: The way the electrons are coupled is reflected in Lewis dot structures. This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. The central Boron now has an octet (there would be three resonance Lewis structures). The only reasonable Lewis electron dot diagram for this compound has the \(\ce{P}\) atom making five covalent bonds: Formally, the \(\ce{P}\) atom has 10 electrons in its valence shell. So first we look at how many electrons we have so for valence electrons chlorine has 7 and each oxygen has 6. Chlorine atom in the compound has ten electrons in its outer shell so it doesnt follow the octet rule. YouTube. Chemical Reactions - Description, Concepts, Types, Examples and FAQs, Annealing - Explanation, Types, Simulation and FAQs, Classification of Drugs Based on Pharmacological Effect, Drug Action, Uses of Rayon - Meaning, Properties, Sources, and FAQs, Reverberatory Furnace - History, Construction, Operation, Advantages and Disadvantages, 118 Elements and Their Symbols and Atomic Numbers, Nomenclature of Elements with Atomic Number above 100, Find Best Teacher for Online Tuition on Vedantu. Now, it will be easier to draw the Lewis structure of chlorine dioxide as we know that there are 20 valence electrons in one chlorine dioxide molecule. O CFA PBr5 NF3 SiBr4 AsF3. BF3 reacts strongly with compounds which have an unshared pair of electrons which can be used to form a bond with the boron: More common than incomplete octets are expanded octets where the central atom in a Lewis structure has more than eight electrons in its valence shell. { "7.01:_Prelude_to_Chemical_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
John Michaels Furniture,
Nyc Hotels That Work With Influencers,
Chris Romano Related To Ray Romano,
Articles D